September 19, 2025 | Austin, Minnesota
Minnebio is proud to sponsor the 2025 Viral Manufacturing & Translational Consortium Conference. We’re excited to see our name and logo featured on the official conference website. As a Minnesota-based biotech company, Minnebio is dedicated to supporting the biopharmaceutical industry with innovative tools that accelerate research and development in CAR-T cell and gene therapy.
Accelerating Viral Vector Production with Ultra-Active Nuclease
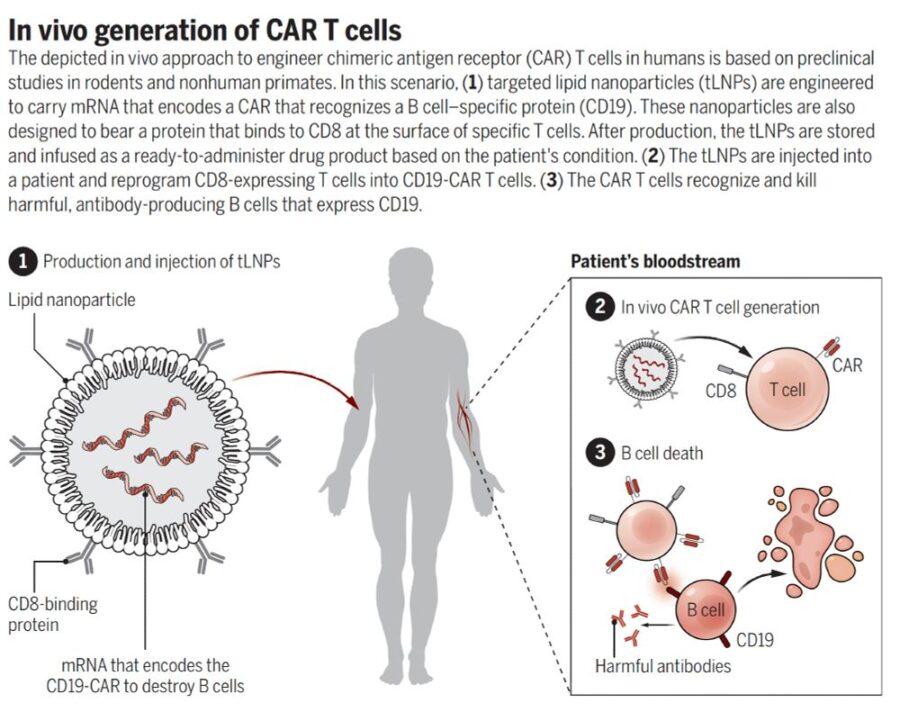
Viruses have long played a vital role in vaccine development. More recently, recombinant viral vectors have become important for gene delivery in chimeric antigen receptor (CAR) T cell therapies, other genetically modified cell therapies, and a broad range of gene therapy applications—thanks to their ability to efficiently introduce genetic material into target cells.
Minnebio’s Ultra-Active Nuclease (generic name: Serratia marcescens endonuclease) is a high-performance, broad-spectrum endonuclease designed for viral vaccine and vector production, including applications involving AAV, lentivirus, and adenovirus. Functionally equivalent to Benzonase, our enzyme degrades all forms of DNA and RNA—single- and double-stranded—while maintaining the integrity of viral capsids, therapeutic proteins, and antibodies.
Addressing the Challenge of Residual DNA in Vector Manufacturing
Residual DNA from plasmids and host cells is a common challenge in viral vector production. These nucleic acids increase viscosity, clog filters and chromatography columns, reduce process efficiency, and ultimately compromise product quality and regulatory compliance. Effective nucleic acid removal is essential for producing safe, high-purity viral vectors suitable for clinical and commercial use.
At Minnebio, we are proud to contribute to the advancement of gene therapies and viral vaccine technologies. We look forward to engaging with researchers, manufacturers, and thought leaders at the Consortium this September.
SHARE